您所在的位置:首页>新闻资讯
公司自成立国际业务部以来,产品逐步打入国际市场,并取得了较好的销售业绩。公司国际业务始终坚持“最好的信誉、最好的服务、 最好的质量”的理念,经过5年的发展,已经在全球客户中建立了良好的信誉,被多家用户评为“最佳供应商”,产品销量年年递增, 并且公司2010年度被医保商会评为中国西成药出口五强企业。
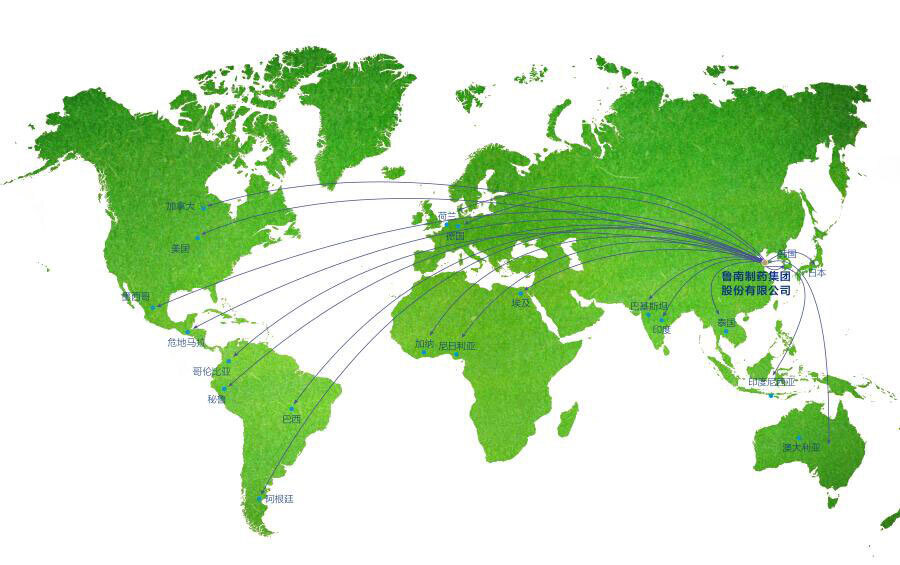
公司产品已销往美国、日本、印度、巴基斯坦、韩国、巴西、埃及、约旦、伊朗、印尼、叙利亚、加拿大、墨西哥、台湾、香港等几十个国家和地区。
在公司的国际化进程中,我们高度重视质量系统的国际化,以ICH和cGMP的标准要求自己,目前公司的cGMP体系已经逐步与欧美接轨,先后通过了哥伦比亚、日本、印尼、巴基斯坦、尼日利亚、韩国等国家的官方现场检查,通过了数十家全球知名客户的供应商审计。
国际注册方面,公司已经取得15个USDMF,1个JDMF,3个KDMF,6个CEP证书,17个印度注册证书等。
公司主要出口产品为:克拉维酸钾系列产品,异氟烷,七氟烷,酶酚酸,4-AA,米格列醇,单硝酸异山梨酯等。